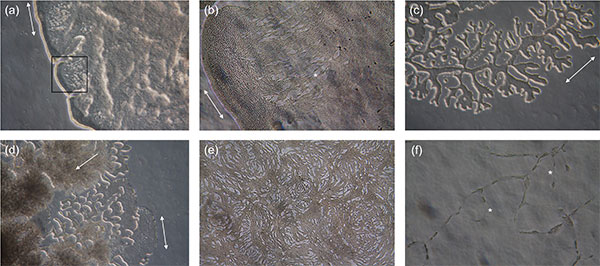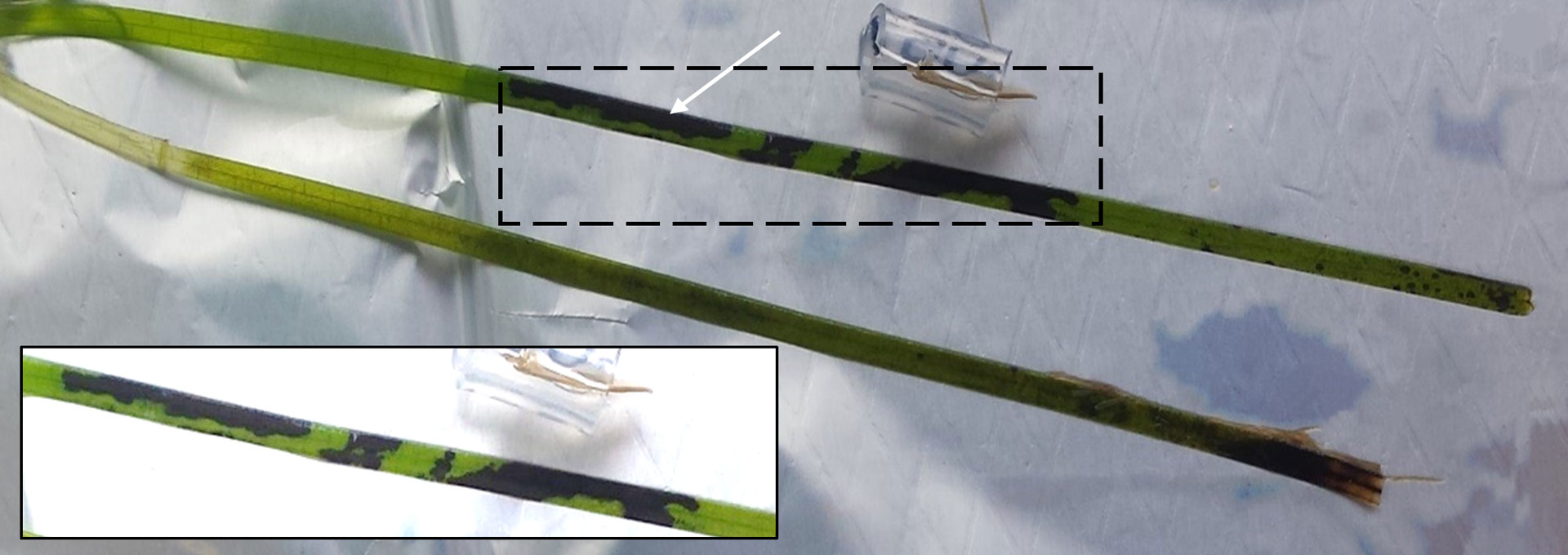
Seagrass Wasting Disease
History in Australia
∗Blue & Bold = Research Gap
Virulence
| Summary | Citation | |
| Opportunistic Pathogens | Typically acts as a saprobe (degrader) of senescent or dead marine plant/algae biomass. However, when isolates can penetrate the epidermis/cell wall of the living host, it can kill the cells, causing black lesions to form. For seagrasses, once enough of the living tissue turns necrotic (i.e. bisect the blade width), nutrient and oxygen resources are blocked and the blade dies. Once enough blades die on a plant or meadow, it may be considered a disease-related die-off. | |
| Abiotic or Biotic Factors | More work needs to be done to understand if there are abiotic or biotic triggers for virulence in Labyrinthula and if so, what are the cellular mechanisms behind them. | |
| Genetics | Primarily as amplicon sequencing for genetic or phylogenetic surveys and identification. There is ongoing work for whole genome sequencing. | NCBI |
| Vectors & Cross-Infection | Known vectors include seagrass blades/wrack as well as the water column. For some Labyrinthula isolates, cross-infection can occur among different seagrass species. | Vergeer & den Hartog 1994; Garcias-Bonet et al. 2011; Martin et al. 2016; Trevathan-Tackett et al. 2018 |
Hosts affected
Reviewed in Martin et al. 2016, Sullivan et al. 2013
Seagrass – Leaves
Macroalgae – Frond/thallus
Mangrove – Leaves
Turf Grass
Ameoba
Fish
Host susceptibility
| Summary | Citation | ||
| Heathy/Neutral Conditions | Labyrinthula has been shown to be present on seagrasses showing no signs of disease, i.e. Older leaves have often been confirmed to be more susceptible to infection than younger leaves. Seagrass leaves that are large and long tend to been shown to have higher incidences of lesions. | Bocklemann et al. 2013; Groner et al. 2014; Groner et al. 2016 | |
| Degraded Conditions | Low salinity | Low salinity environments typically act as a refuge against infection due to Labyrinthula intolerance to low salinities (typically <15 psu). Even if seagrasses are stressed by low salinities, it is not expected to result in higher incidences of wasting disease. | Jakobsson-Thor et al. 2018 |
| High salinity | It is suggested that high salinity alone cannot cause increases in seagrass wasting disease, possibly linked to the metabolic response of seagrass to high salinity (increase respiration and ROS) indirectly slowing Labyrinthula growth and infection. | Trevathan et al. 2011 | |
| High temperature | Presence of Labyrinthula zosterae and symptoms of wasting disease has been shown to be higher in summer months in field studies. High temperature stress, in some cases, has been hypothesised to cause stress in seagrass and potentially higher susceptibility to disease. | Bocklemann et al. 2013 | |
| Light | Insufficient research, although it is hypothesised that reduced photosynthesis would reduce the health of the seagrass and thus cause it to be more susceptible to disease. | Vergeer et al. 1995 | |
| Eutrophication | Insufficient research | NA | |
| Depth | There is contradictory evidence on the effect of depth on wasting disease. Both relative shallow and deep meadows have been shown to have increased Labyrinthula prevalence. | Groner et al. 2014; Jakobsson-Thor et al. 2018 | |
| Multiple stressors | Temperature + salinity + oxygen sulphide stress | The effect of multi-stressor events on seagrass wasting disease are complex. Elevated infection/disease is likely only to occur if the seagrass is immunocompromised and Labyrinthula is not. In some cases, environmental stress can negatively affect both host and pathogen resulting in low infection/disease rate. | Bishop et al. 2017 |
| Salinity + depth | Infection increased with salinities > 25 psu and with increasing depth. | Jakobsson-Thor et al. 2018 | |
| Potential for defence or recovery | Secondary metabolites | Production of biochemical compounds by seagrass has been suggested defence mechanisms for Labyrinthula infection, either directly or indirectly and in vitro. Potential compounds include phenolic acids, tannins, flavone glycosides. It is hypothesised that seagrasses have both constitutive (innate) and induced (produced) biochemical defences. | Vergeer & Develi 1997; Arnold & Targett 2002; Steele et al. 2005; Brakel et al. 2014; Trevathan-Tackett et al. 2015; Jakobsson-Thor et al. 2018 |
| Reactive oxygen species | Reactive oxygen species (ROS) and the hypersensitive response is a mechanism used seagrasses, likely as a defence response. There is evidence that seagrasses accumulate ROS during infection, but it is unclear if Labyrinthula presence or invasion into the tissues directly triggers this response. | Trevathan et al. 2011; Loucks et al. 2013 | |
| Limitation to lesion growth | Lesion prevalence may be high in seagrass meadows, yet meadows generally do not commonly experience wasting disease-related die-backs. It is not clear the exact mechanism of the limited infection, whether it be linked to seagrass defences or limited capacity of some Labyrinthula isolates to infect living, healthy tissue. | NA | |
Environment
| Summary | Citation | ||
| Disease Inhibition | Low salinity | Low marine/estuarine salinities are not suitable conditions for Labyrinthula; typically, salinities below 10-15 psu. | McKone & Tanner 2009 |
| High salinity | Short-term hypersalinity events (> 40-45 psu) have been shown to suppress Labyrinthula proliferation and infection. | Trevathan-Tackett et al. 2011; Bishop et al. 2017 | |
| Disease Proliferation | Virulence genes | Mechanisms behind Labyrinthula virulence is a topic still undergoing research. | NA |
| Seagrass density | Seagrass meadows with high leaf density have been show to exhibit higher degree of lesions and diseases, likely due to the great chance of leaf-to-leaf transference of pathogenic Labyrinthula | Groner et al. 2016; Irving et al. 2016 | |
| Climate Change & Future Predictions | There has been some work predicting the effects of climate change on seagrass wasting disease. Most are laboratory experiments simulating shifts in environmental conditions (see above), while some papers have taken a modelling approach to predict disease and transference. | Irving et al. 2016 |
Identification
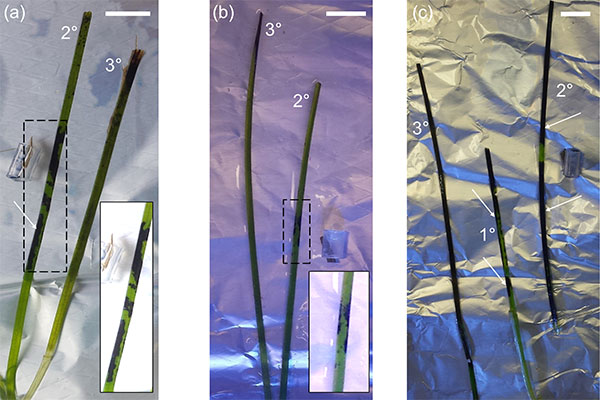
Photo credit: Trevathan-Tackett et al. 2018
Photo credit: Trevathan-Tackett et al. 2018
Monitoring
| Method of detection | Pros of Method | Cons of Method | Notes | Citation |
| Lesions | Easy; Technically inexpensive | Manually intensive; Assumes all lesions are caused by Labyrinthula infection | Still used for initial ID with other methods to confirm disease | Burdick et al. 1993 |
| Culturing | Best standardised way to ID Labyrinthula presence; Best way to build up culture for genetic sequencing or laboratory research; Media can be made inexpensively | Isolation and maintaining cultures is labour intensive; Some media recipes are expensive; Need to have training in Labryinthula identification and sterile technique; Cultures can be prone to die-off or have fungal contamination if frequent sub-culturing is not maintained | Cryopreservation can be used to keep stock cultures but still unknown how genetics or virulence is affected | Martin et al. 2009; Muehlstein et al. 1991; Trevathan et al. 2011; Trevathan-Tackett et al. accepted in Disease of Aquatic Organisms |
| Pathogenicity/Koch’s Postulates | Best standardised way to ID virulence characteristics of Labyrinthula and host susceptibility in a controlled environment | Requires living Labyrinthula cultures, mesocosm facilities and proper waste methods to prevent spread of pathogenic Labyrinthula beyond test subject | Need to consider natural Labyrinthula presence (and infection) on the healthy seagrass hosts used in testing (i.e. acclimation period prior to infection) | Short et al. 1987; Martin et al. 2016 |
| (q)PCR | Rapid identification and/or quantification of Labyrinthula on host | Requires specific laboratory facilities and instrumentation; Knowledge of genetics and PCR techniques needed; Methodology and primer development are still being optimised | Primers used for L. zosterae (q)PCR may need to be tested and adapted to local Labyrinthula isolates | Bocklemann et al. 2013 |
Prevention
Tested Method of Prevention: TBD
Management
Tested Method of Prevention: TBD
Risk Assessment
∗Blue & Bold = Research Gap
Natural Occurrence in Australia
| Location | Present | Level of Consequence | Level of Likelihood | Risk Analysis | Risk Evaluation | Justification of Score & Research Gaps | Data Sources |
| NSW | Y | Minor | Unlikely | 2 | Negligible | Labyrinthula sp. detected on seagrass leaf blades, with and without disease symptoms in 2014-2015. Genetic testing indicate the isolates group with other non-pathogenic Labyrinthula clades. Current data are limited to the Sydney region. | Sullivan et al. 2017, doi:10.1111/jeu.12387 |
| VIC | Y | Moderate | Unlikely | 4 | Low | Labyrinthula sp. detected on seagrass leaf blades, with and without disease symptoms in 2016. Genetic testing indicated the isolates group with both pathogenic and non-pathogenic Labyrinthula clades. Laboratory pathogenicity test confirmed virulence, but disease effect on natural populations still unknown | Trevathan-Tackett et al. 2017, doi: 10.1016/j.micres.2017.10.003 |
| WA | Y | Insufficient data | Insufficient data | NA | NA | Labyrinthula sp. detected on seagrass leaf blades, with and without disease symptoms in the early 1990s. No pathogenicity tests were performed and no published studies have been performed since | Vergeer & den Hartog 1994, doi: 10.1016/0304-3770(94)90070-1 |
| QLD | Y | Insufficient data | Insufficient data | NA | NA | Labyrinthula sp. detected on seagrass leaf blades, with and without disease symptoms in 2009. Genetic tests indicated it was part of the pathogenic clade of Labyrinthula but pathogenicity tests were inconclusive. | Martin et al. 2016, doi: 10.1007/s12237-016-0087-z |
| SA | NA | ||||||
| NT | NA | ||||||
| TAS | NA |
Introduction into new areas, cultures, stocks
Sources
| Sources | Present | Level of Consequence | Level of Likelihood | Risk Analysis | Risk Evaluation | Justification of Score & Research Gaps | Data Sources |
| Water | NA | ||||||
| Sediment | NA | ||||||
| Run-off | NA | ||||||
| Host | Y | Minor to Moderate | Likely | 4 to 8 | Low to Moderate | Labyrinthula sp. Is considered a ubiquitious host-associated microorganism. See 'Disease Ecology' tab for information on elevated virulence or disease risk | Vergeer & den Hartog 1994, doi: 10.1016/0304-3770(94)90070-1 |
| Non-host species | Y | Minor to Moderate | Possible | 3 to 6 | Low to Moderate | Australian Labyrinthula isolates have been shown to be able to cross-infect or be isolated from more than one seagrass species, and globally seaweeds, but resulting disease has been variable and must be considered on a case-by-case basis | Trevathan-Tackett et al. 2017, doi: 10.1016/j.micres.2017.10.003; Sullivan et al. 2016, 10.1016/j.funeco.2013.06.004 |
| Humans | N | No evidence in the literature that Labyrinthula has been introduced directly from humans |
Vectors
| Vectors | Present | Level of Consequence | Level of Likelihood | Risk Analysis | Risk Evaluation | Justification of Score & Research Gaps | Data Sources |
| Water | Y | Major | Unlikely | 6 | Moderate | Labyrinthula sp. Has been seen to infect seagrass leaves when cultures were applied to water in laboratory settings, but long-dispersal (including inter-continental) via water is unknown/debated | Martin et al. 2016, doi: 10.1007/s12237-016-0087-z |
| Sediment | NA | ||||||
| Run-off | NA | ||||||
| Host | Y | Major | Unlikely | 6 | Moderate | As seagrass leaves are the known preferred location for Labyrinthula, scenescent floating leaves are most likely the most common form transference within and among seagrass populations | |
| Non-host species | Y | Insufficient data | Insufficient data | Labyrinthula has been found to exist on non-seagrass seaweeds and animals, so while it is likely to be present, there is no data on the occurrence of Labyrinthula on non-seagrass hosts in Australia | |||
| Humans | NA |
Spread of disease
| Present | Level of Consequence | Level of Likelihood | Risk Analysis | Risk Evaluation | Justification of Score & Research Gaps | Data Sources | |
| Rate of infection: | Y | Major | Likely | 12 | High | No studies have been performed on rate of disease proliferation in natural populations in Australia. Laboratory tests suggest that virulent isolates can rapidly spread across whole leaves and spread of other leaves of the same plant within a week's time | Trevathan-Tackett et al. 2017, doi: 10.1016/j.micres.2017.10.003 |
| Spread outside of population, culture, stock: | NA | Large-scale dispersal is unknown for Labyrinthula in Australia. See 'Disease Ecology' tab for global examples. |
How to read the Risk Tables
Risk Marix1:
| Likelihood Level | |||||
| Consequence Level | Remote | Unlikely | Possible | Likely | |
| 1 | 2 | 3 | 4 | ||
| Minor | 1 | 1 | 2 | 3 | 4 |
| Moderate | 2 | 2 | 4 | 6 | 8 |
| Major | 3 | 4 | 6 | 9 | 12 |
| Severe | 4 | 3 | 8 | 12 | 16 |
1Levels of Consequence:
Minor: measurable, but minor levels w low/no impact
Moderate: some impact
Major: reduction and impact on reproduction
Severe: significant reductions in size and reproduction
Risk Evaluation1:
| Risk Level | Risk Score | Description |
| Negligible | 0 to 2 | With current data, acceptible with no management actions or regular monitoring. Research gaps may remain. |
| Low | 3 to 4 | Acceptable with no direct management actions; monitoring at specific intervals. Research gaps need addressing. |
| Moderate | 6 to 8 | Acceptable with specific, direct management and regular monitoring. Pressing research gaps |
| High | 9 to 16 | Unacceptable unless additional management actions are undertaken, possibly for recovery. Critical research gaps. |
1Levels of Likelihood:
Remote: Consequence not hear of but plausible
Unlikely: Not likely, unless in special circumstance
Possible: May occur in some circumstances
Likely: Consequence is expected to occur
1Assuming no management methods are taken. Modified from Department of Fisheries Western Australia, ‘Threat Identification, Hazard Pathway Analysis and Assessment of the Key Biosecurity Risks presented by the establishment of the Mid-West Aquaculture Development Zone in Western Australia, August 2015